Pollination Biology of Symphonia globulifera (Clusiaceae) in central French Guiana 1


Copyrighted 1998 by the Association for Tropical Biology, P.O. Box 1897, Lawrence, KS 66044-8897. Reprinted by permission from Biotropica 30(1): 139-144. 1998.
Key words: Clusiaceae, hummingbirds, nectar, ornithophily, perching birds, pollination biology, Symphonia globulifera.
Recent reports on the pollination of Symphonia globulifera L. f. (Clusiaceae) have not clearly established the relative importance of hummingbirds and perching birds in the pollination of this species. Pascarella (1992) observed that hummingbirds visit the flowers of S. globulifera in Costa Rica, but he concluded that hummingbirds are probably nectar robbers and not pollinators. He suggested that other visitors, particularly Lepidoptera, may be the pollinators. In contrast, Bittrich and Amaral (1996) suggested, with some reservation, that in central Amazonian Brazil S. globulifera is pollinated mainly by hummingbirds. The purpose of this note is to demonstrate that perching birds play an important role in the pollination of S. globulifera, at least in central French Guiana.
Symphonia globulifera is a widespread species found in southern Mexico, most of Central America, parts of the West Indies, Amazonian and Guayanan South America, and tropical West Africa. Although only one species is currently recognized in the Neotropics and tropical West Africa, as many as 16 taxa of Symphonia may be present in Madagascar (Maguire 1964). There is considerable morphological variation in the Neotropical populations of S. globulifera. For example, in Central America it occurs mostly as a canopy tree in swampy habitats, but at La Selva in Costa Rica it is usually an understory tree less than 15 m tall (Hammel 1986). In French Guiana, Loubry (1994) suggested that what is treated as a single species may in fact be two species distinguished by morphological and ecological features. He applied the name S. globulifera to a morphologically distinct entity found in periodically flooded habitats and designated individuals growing on non-flooded forest (terra firme) as Symphonia sp. Although we use S. globulifera in the broad sense, the individuals we studied all grow on terra firme and correspond to Symphonia sp. sensu Loubry.
This study was conducted from the 8 —23 September 1994 in central French Guiana near Eaux Claires, a small homestead located approximately 4.5 km NNW of the village of Saül (3° 37'N, 53° 12'W). The area is mostly covered by lowland moist forest and is described in detail by Oldeman (1974) and Mori and Boom (1987). Central French Guiana experiences a distinct dry season from August to November (Mori & Prance 1987).
Four trees of S. globulifera (ranging from 17-30 m tall ´ 18-26.5 cm DBH) were selected for study based on availability of flowers and ease of climbing. The trees, identified by their nos. Mori et al. 23736, 23852, 23999, and 24000, are vouchered at NY. Most of the observations of floral visitors were made in tree 23852.
Flowers at different stages of development were examined in order to establish the relative positions of the floral parts from just before anthesis to the time the petals dropped. In addition, flower structure was studied using ETOH preserved flowers. Flower color was recorded according to Kornerup and Wanscher (1984).
Flower longevity was determined by marking 90 buds on several branches of one tree. As new flowers opened, i.e., when the petals relaxed to expose the staminal tube, they were observed hourly until the petals dropped from the flower. We define this period of time as the life of the flower, but did not determine the exact timing of anther dehiscence and stigma receptivity.
Nectar was removed from the flowers during the morning using 10 µl graduated microcapillary tubes, and the samples were scored for the amount of nectar available and analyzed for total sugar concentration or "sucrose equivalents" with a hand held refractometer (Milton Roy model 39-45-01).
Nectar samples from each tree were blotted onto Whatman's no. 1 filter paper. These samples were later analyzed for sucrose, glucose, and fructose content at the University of Connecticut Biotechnology Center, Storrs, CT. The sugar contents were determined using standard High Pressure Liquid chromatography (HPLC) techinques.
Preliminary observations were made on flower visitors each time the trees were climbed. Trees were climbed several times during the night and observations were made for several hours in an effort to record the presence of nocturnal flower visitors. Because these observations indicated that birds were most likely the principal visitors to the flowers of Symphonia globulifera, diurnal bird visits to flowers were recorded for a total of slightly over 24 hours on three separate days as follows: day one from 0630 to 1830 h, day two from 1345 to 1930 h, and day three from 0630 to 1215 h and from 1645 to 1930 h. During this series of observations, 45 minutes of the hour were spent recording the first time a bird entered the tree and inserted its bill into a flower. Subsequent visits by the same bird to other flowers were ignored. The bird visits were classified as either a perching bird visit or a hummingbird visit. The other 15 minutes of the hour were used for identifying birds, resting, and changing of observers.
The inflorescences of Symphonia globulifera are axillary and consist of 1 to 17 flowers. At any given time, 1-3 flowers are at anthesis in an inflorescence. The flowers are arranged such that they face upward and can easily be reached from the branches (Fig. 1A). The odorless flowers are globose to depressed globose and 10-12 mm in diameter at anthesis. The five contorted, strongly imbricate, cucullate petals form a chamber in which nectar accumulates (Figs. 1C-E). The color of the petals in bud and for most of the length of the visible part of the petals at anthesis is high red (Methuen color 10A8) except for their distal quarter which is wine red (11D8). The filaments are fused into a tube with five pastel red (9A4) lobes at the apex. The staminal tube closely surrounds the pistil (Figs. 1E-F). The foliage yellowish-green (30A6) stigma is shaped like a five-lobed star (Fig. 1C). Small pores are found at the apices of each of the lobes of the stigma (Bittrich & Amaral, 1996). At anthesis, the anthers and the stigma extend above the level of the petals and each lobe of the stigma projects outward between the two adjacent anthers such that the stigmatic surfaces and the anthers are at the same level (Fig. 1F). The anthers are adnate to the lobes of the staminal tube and open abaxially (Figs. 1F, 1H) to display pollen immersed in a sticky, glue-like substance. A well-developed nectary surrounds the staminal tube at its base (Figs. 1E-F).
|
|
|
Figure 1. Symphonia globulifera (A-H, Mori et al. 23852; I, Oldeman 2019A): A - stem with leaves and inflorescences, B - detail of leaf venation, C - lateral (above left) and apical (below right) views of flowers, D - cucullate petal, E - medial section of flower, note staminal tube, F - flower with petals removed, note staminal tube, anthers, and stigmatic lobes between lobes of staminal tube, G - pistil (left), apical view of stigma (above left), and transverse section of ovary (right), H - lateral (far left) and abaxial (near left) views of lobes of staminal tube with adnate anthers, I - fruit (right) and seeds (left). |
Some flowers opened within minutes whereas others were observed to take up to one and one-half hours to open. In the process of opening, the petals bulged outward causing them to slide down the staminal tube rather than the staminal tube and style growing up through the corolla. As this process continued, a small gap of ca. 1 mm developed between the petals and the staminal tube thereby allowing access to the nectar. At anthesis, the stigma and the anthers projected ca. 3 mm above the corolla. At the end of anthesis, the petals dropped one by one.
Mean flower longevity (n = 90) was 31.1 ± 1.2 h with the shortest lived flowers persisting for 30.1 h and the longest lived flowers lasting for 32.2 h. Flowers generally opened at around 2000 h and the petals fell between 0200 and 0400 h some 30-32 hours later. The flowers are therefore available to potential pollinators during the night as well as during the day.
Overall nectar production ranged from 3.0-130 µl per flower. Because our samples were gathered from open flowers, this variation can be attributed to a combination of inter-tree variation, local environment, and differences in removal of nectar by birds. Nevertheless, a given flower may provide a substantial amount of nectar to pollinators, especially if the flower has not been previously visited.
Total sugar concentrations ranged from 5.0 to 17.0 percent for all flowers sampled. The means of the individual trees ranged from 8.8 percent in tree 23736 to 13 percent in tree 23852 (Table 1).
| TABLE 1. Total sugar concentration (mean of 30 flowers) and sugar type (based on a single sample per tree) of the floral nectar of four trees of Symphonia globulifera. m.w. = molecular weight. | Sugar type and concentration | Tree 23736 | Tree 23852 | Tree 23999 | Tree 24000 |
| total sugar conc. | 8.8% ± 1.02 | 13.0% ± 1.14 | 9.8% ± 1.30 | 9.0% ± 1.92 |
|
high m.w. sugar |
5.26% |
5.41% |
4.87% |
3.04% |
|
sucrose |
2.72% |
2.13% |
9.47% |
0% |
|
glucose (hexose) |
46.58% |
47.74% |
41.15% |
49.96% |
|
fructose (hexose) |
45.44% |
44.72% |
43.69% |
45.10% |
|
unknown |
0% |
trace |
0.82% |
1.89% |
The nectar composition was hexose-dominant in trees 23736, 23852, and 24000 and hexose-rich in tree 23999 (hexose = glucose plus fructose, see Baker & Baker 1983 for definitions of sugar dominance). The percent glucose ranged from 41.15 to 49.96 and the percent fructose from 43.69 to 45.44. In contrast, the sucrose percentage ranged from 0 to 9.47 (Table 1).
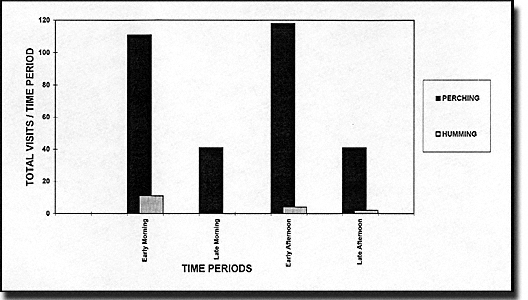
Visits to the flowers of Symphonia globulifera were dominated by perching birds during all times of the day (Fig. 2). The most frequent and persistent perching birds visiting the flowers were five species of Thraupidae (including part of the Coerebidae according to Meyer de Schauensee & Phelps 1978). These birds (the black-faced dacnis, blue dacnis, green honeycreeper, purple honeycreeper, and red-legged honeycreeper), which are among the most colorful in this forest, usually visited the trees as members of mixed flocks. Three of the occasional perching bird visitors were also Thraupidae (the fulvous-crested tanager, paradise tanager, and silver-beaked tanager); one, the bananaquit, is a Parulidae, and another, the waved woodpecker, is a Picidae (see Meyer de Schauensee & Phelps 1978 for corresponding scientific names of the birds). Although hummingbirds visited the flowers, their visits were so brief that we did not make confident determinations. No insects were seen to be consistent and persistent visitors nor were any nocturnal animals observed taking nectar from the flowers of S. globulifera. However, the presence of open flowers at night dictates that more extensive nocturnal observations be made before the possibility of bats and moths are excluded as potential pollinators. However, lack of floral aroma and red flower color are usually not associated with bat and moth pollination.
|
|
Figure 2. Diurnal visits by perching birds and hummingbirds to the flowers of a tree of Symphonia globulifera during a 24-hour observation period in September 1994 in central French Guiana. The bars represent the total number of perching bird and hummingbird visits for each time period. |
Although we were not able to confirm the presence of an initial male phase in the flower as described by Pascarella (1992), our observations do not contradict the possibility of male phase flowers. At our study site, the flowers of S. globulifera dehisced anthers very early in anthesis, but we did not determine if the stigmas were also receptive at the same time. Because of the juxtaposition of the anthers and the stigma (Fig. 1F), the presence of a separate male phase would favor fertilization resulting from the transfer of pollen from one flower in the male phase to another in the female phase.
The position of the flowers (which are easily reached by birds perching on the branches), the diurnal availability of the odorless flowers, the red corolla forming a nectar chamber, the ovary protected by the staminal tube, the position of the anthers and the stigmatic surfaces such that they are touched by birds taking nectar, the pollen embedded in a glue-like substance (antheroil fide Bittrich & Amaral, 1996) that readily adheres to birds' beaks, the relatively low sugar concentrations in the nectar (Table 1), and the hexose-dominant or hexose-rich nectar (Table 1) coupled with our observations of perching birds as consistent visitors to the flowers suggest that perching birds are important pollinators of S. globulifera in central French Guiana. In particular, it appears that members of the Thraupidae are the dominant birds in this relationship in central French Guiana. Species of Thraupidae are widely reported to take nectar and may even feed extensively on nectar during certain times of the year (Isler & Isler 1987).
We conclude, as did Pascarella (1992), that hummingbirds are incidental visitors or even nectar robbers of S. globulifera. We do not agree, however, with his suggestion that lepidoptera may play an important role in its pollination. He makes this statement based on the co-occurence of lepidoptera and S. globulifera throughout the range of the latter. Perching birds, although of different families, are found in the lowlands of both the Neotropics and Africa (Stiles 1981) and therefore could play a role in the pollination of S. globulifera in both areas. Offutt (1990) has observed that the flowers of S. globulifera serve as a source of food for birds in West Africa. Although hummingbirds visit, and may even defend trees of S. globulifera from other hummingbirds, this behavior may be less common in trees occuring in undisturbed forest where we made our observations than it is in the disturbed habitat in central Amazonia where Bittrich and Amaral (1996) carried out their study.
Many of the dominant and occasional perching birds that we observed visiting the flowers of S. globulifera in central French Guiana are known to forage for nectar and fruit in the canopy (Meyer de Schauensee 1978). Hummingbirds, on the other hand, are not common pollinators of canopy trees (Stiles 1978, 1981).
Symphonia globulifera belongs to a presumably natural assemblage of genera found principally in the Neotropics. This group, consisting of Lorostemon, Moronobea, Platonia, Symphonia, and Thysanostemon, has been treated as subfamily Moronoboideae and is distinguished by 1) aggregation of the stamens into five discrete phalanges, 2) indehiscent, baccate fruit, and 3) comparatively few, large seeds without arils (Maguire 1964). Bittrich and Amaral (1996) point out the similarity between Platonia and Symphonia in their common possession of antheroil and stigmas with apical pores. We add that the genera of Moronoboideae possess 1) distichous leaves, 2) axillary flowers easily reached by birds from the branches, and 3) floral nectar —all features which suggest that perching bird pollination may be widespread in this subfamily of Clusiaceae. This hypothesis is supported by the observation of small "euphonia-like" birds visiting the flowers of Moronobea coccinea Aublet (vouchered by Mori & Boom 15266 at CAY and NY). We conclude that future studies of the reproductive biology of Symphonia and its relatives should not neglect the role of perching birds in their pollination.
We are grateful to Carol Gracie and Rob Jones for help in the field and to Victor Albert, Amy Berkov, William R. Buck, Carol Gracie, and John D. Mitchell for their reviews of the manuscript. We thank Bobbi Angell for the illustration of Symphonia globulifera. We are thankful to Thomas Philbrick for his guidance in the first draft of this manuscript which served as the senior research project of Gill and Fowler at Western Connecticut State University. Field work for this study was supported by a National Science Foundation Research Experiences for Undergraduates supplement to Scott A. Mori's NSF grant BSR-9024530 (Botanical Inventory of a French Guiana Forest) and by The Fund for Neotropical Plant Research of The New York Botanical Garden through a grant from the Beneficia Foundation.
Baker, H. G. and I. Baker. 1983. Floral nectar sugar constituents in relation to pollinator type. In C. E. Jones and R. J. Little (eds.). Handbook of experimental pollination biology, pp. 117-114. Scientific and Academic Editions, Division of Van Nostrand Rheinhold Company Inc., New York.
Bittrich, V. & M. C. E. Amaral. 1996. Pollination biology of Symphonia globulifera L. f. (Clusiaceae). Pl. Syst. Evol. 200: 101-110.
Hammel, B. E. 1986. The vascular plant flora of La Selva Biological Station, Costa Rica: Guttiferae. Selbyana 9:20-3-217.
Isler, M. L. and P. R. Isler. 1987. The tanagers: Natural history, distribution, and identification. Smithsonian Institution Press, Washington, D.C. 404 pp.
Kornerup, A. and J. H. Wanscher. 1984. Methuen handbook of color. Methuen, London, England.
Loubry, D. 1994. Déterminisme du comportement phénologique des arbres en forêt tropical humide de Guyane française (5° lat. N.). Thèse de Doctorat de l'Université Paris 6, Specialité: Biologie Végétale Tropicale.
Maguire, B. M. 1964. Guttiferae subfam. Moronoboideae (American genera). In B. M. Maguire & J. J. Wurdack, The botany of the Guayana Highland -- Part V. Mem. New York Bot. Gard. 10(5): 123-135.
Meyer de Schauensee, R. and W. H. Phelps. 1978. Birds of Venezuela. Princeton University Press, Princeton, New Jersey.
Mori, S. A. and B. M. Boom. 1987. The Forest. In S. A. Mori (Ed.). The Lecythidaceae of a lowland Neotropical forest: La Fumée Mountain, French Guiana. Mem. New York Bot. Gard. 44: 9-29.
_______ and G. T. Prance. 1987. Phenology. In S. A. Mori (Ed.). The Lecythidaceae of a lowland Neotropical forest: La Fumée Mountain, French Guiana. Mem. New York Bot. Gard. 44: 124-136.
Offutt, K. 1990. Nyungwe forest reserve. Wildlife Conservation International NYZS and the Ruwandan Office of Tourism and National Parks (ORTPN).
Oldeman, R. A. A. 1974. L'architecture de la forêt guyanaise. Mém. ORSTOM 73: 1-204.
Pascarella, J. B. 1992. Notes on flowering phenology, nectar robbing and pollination of Symphonia globulifera L. f. (Clusiaceae) in a lowland rain forest in Costa Rica. Brenesia 38: 83-86.
Stiles, G. F. 1978. Ecological and evolutionary implications of bird pollination. Amer. Zool. 18: 715-727.
_______. 1981. Geographical aspects of bird-flower coevolution, with particular reference to Central America. Ann. Missouri Bot.Gard. 68: 323-351.
George E. Gill, Jr.2, Ryan T. Fowler3
Department of Biology, Western Connecticut State University, Danbury, CT 06810, U.S.A.
AndScott A. Mori4
Institute of Systematic Botany, The New York Botanical Garden, Bronx, New York 10458-5126, U.S.A.
1Received 16 December 1995; revision accepted 10 October 1996.
2Present address: 4813 B. Tapers Dr., Raleigh, North Carolina 27604.
3Present address: 486 Quaker Farms Road, Oxford, Connecticut 06478.
4Address all correspondence to S. A. Mori at The New York Botanical Garden.